
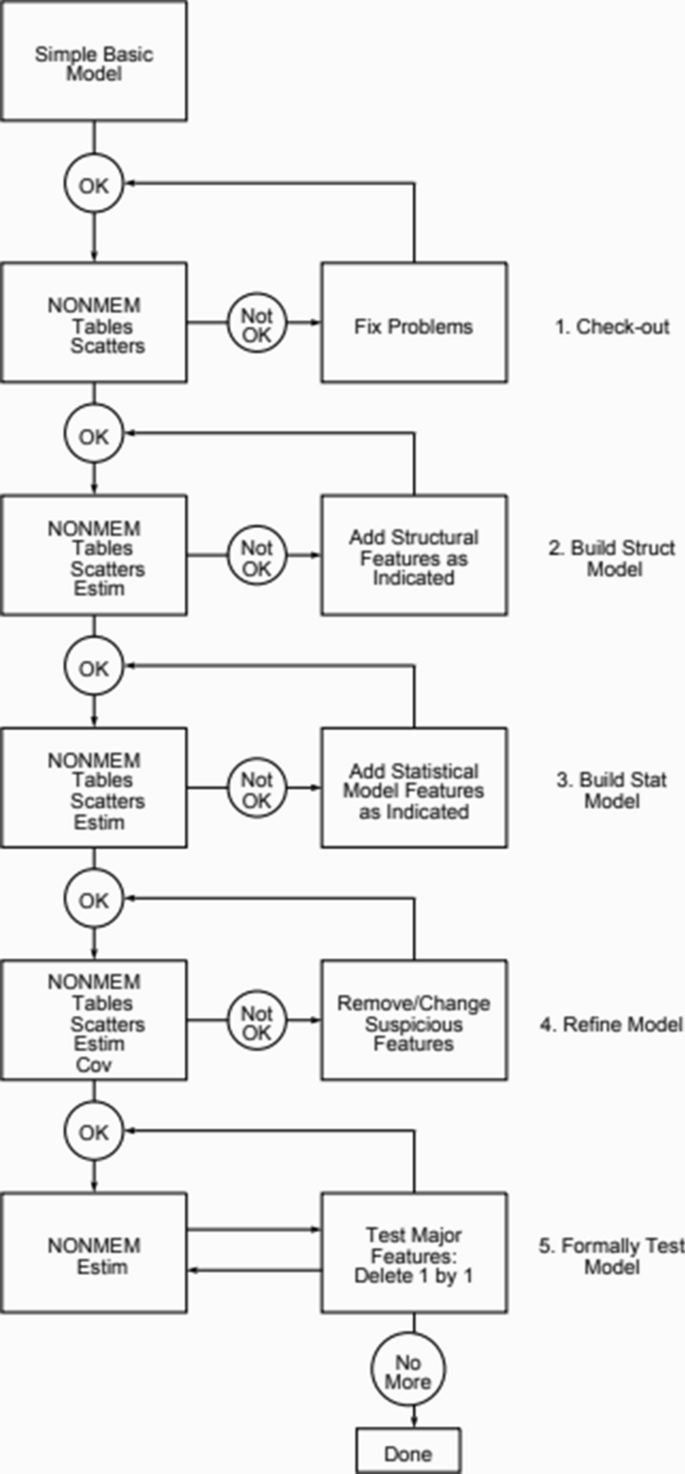
#What is nonmem full
A patient‐friendly alternative to full AUC measurements, which requires intensive sampling, is a limited‐sampling strategy (LSS), based on 3 or 4 time points. Nevertheless most centres use trough levels as it is less complicated to perform. It is well known that the area‐under the concentration–time curve (AUC) is the best link between exposure and effect, superior to trough concentration measurements. Further dose optimization is guided by therapeutic drug monitoring (TDM). To achieve adequate exposure early after transplantation, transplant recipients can be genotyped for CYP3A5 polymorphisms in order to adjust the initial starting dose. In contrast to this large PK variability, the therapeutic window of tacrolimus is relatively small while subtherapeutic exposure increases the risk of graft rejection, supratherapeutic exposure may lead to side effects such as renal toxicity, diabetes, leukopenia and tremors. IL levels and IL‐10, IL‐6, IL‐18 and tumour necrosis factor‐α polymorphisms have been associated with altered tacrolimus PK. Cytochrome P450 enzymes CYP3A4 and CYP3A5, impacting first‐pass metabolism and thereby bioavailability.ĭrug–drug interactions, food intake and clinical conditions such as diarrhoea also influence the exposure to tacrolimus.įurthermore evidence is accumulating that proinflammatory cytokines are able to down‐regulate CYP enzymes.
This variability can be partly explained by genetic differences in metabolizing capacity of enzymes present in the epithelial wall of the gut and in the liver, i.e. Tacrolimus has a large interindividual variability (IIV) in pharmacokinetics (PK). In the long term, tacrolimus is frequently combined with mycophenolate mofetil or other immunosuppressants and the dose is reduced to decrease the risk of renal toxicity. Shortly after transplantation, in most centres tacrolimus is combined with induction therapy with the IL‐2‐receptor antagonist basiliximab and prednisone. Tacrolimus inhibits the T‐cell mediated immune response by blocking the synthesis of interleukin (IL) and is now the cornerstone in the immunosuppressive regimen post‐liver transplantation. The introduction of the calcineurin inhibitors cyclosporine and tacrolimus has improved survival of patients after liver transplantation dramatically.


 0 kommentar(er)
0 kommentar(er)
